Abstracts and Manuscripts
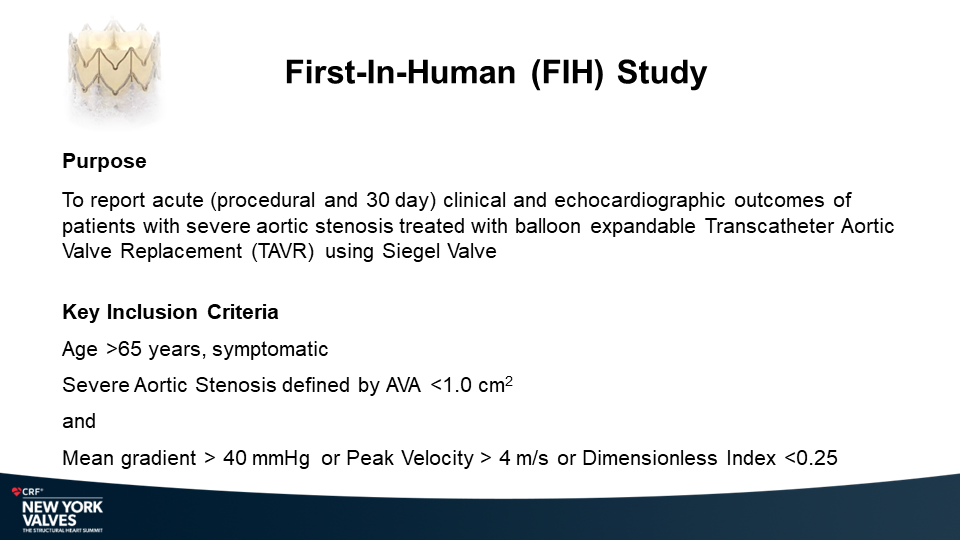
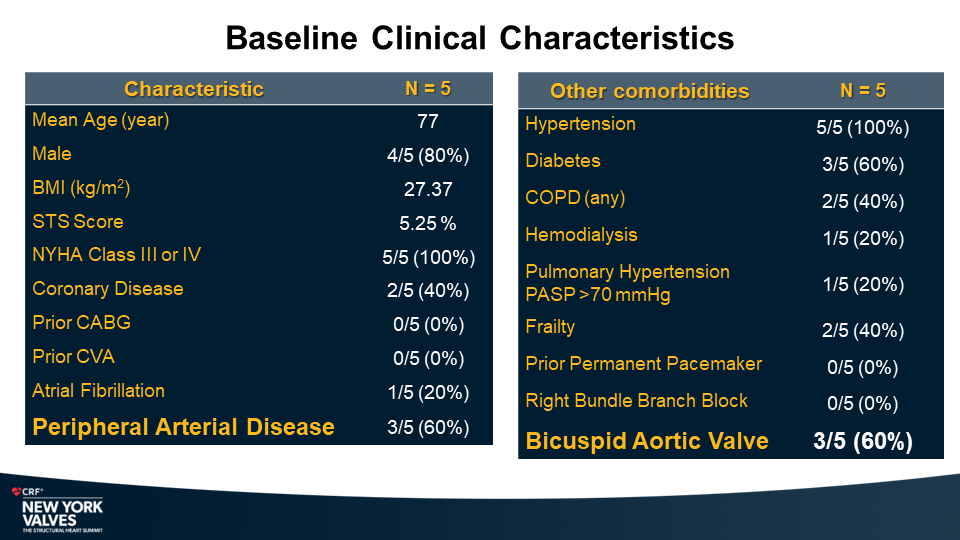
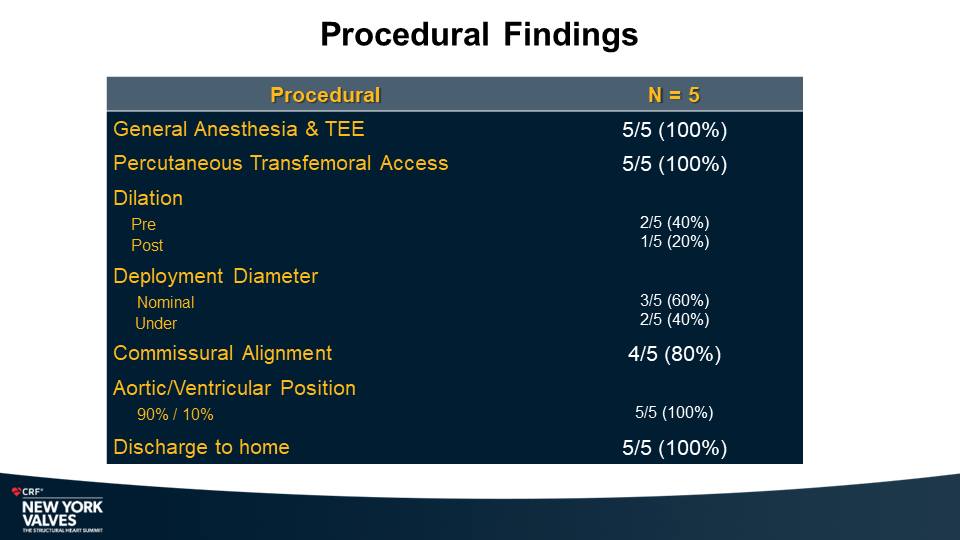
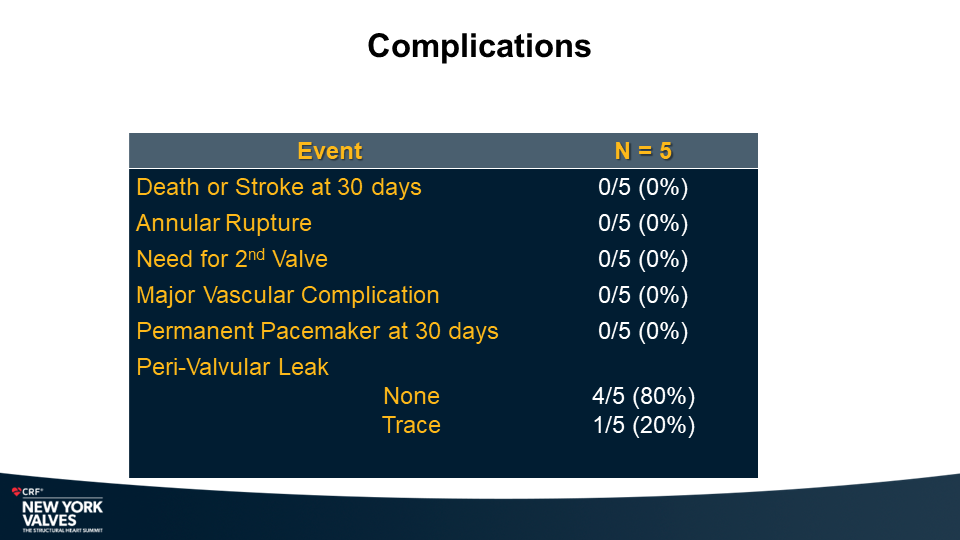
MiRus Siegel™ TAVR System FIH Results
Yadav P., et.al
NY Heart Valves Annual Meeting.
June 6, 2024; New York, NY


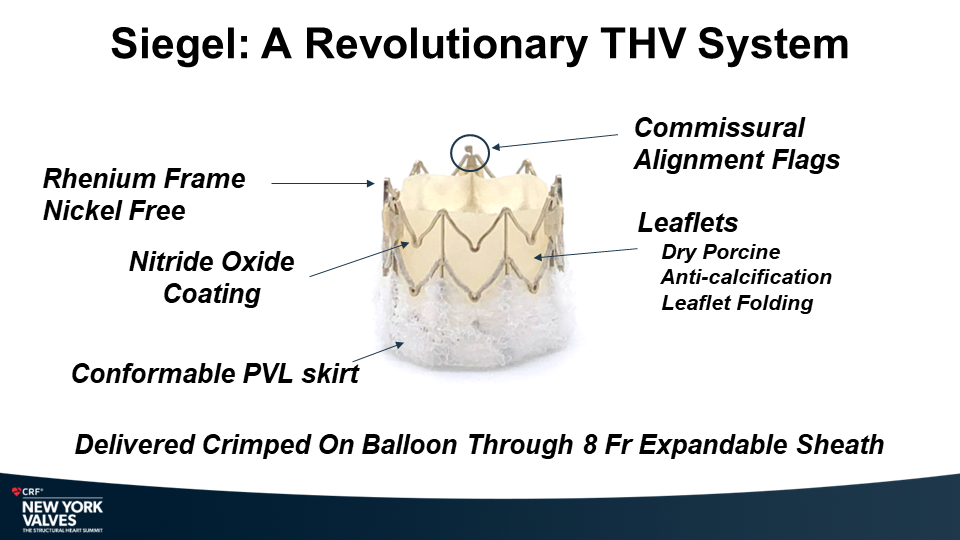
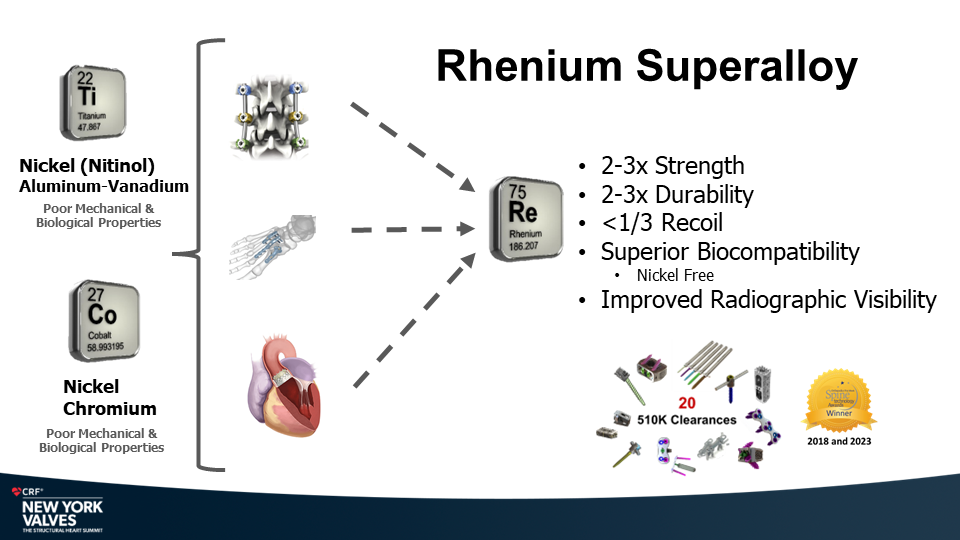
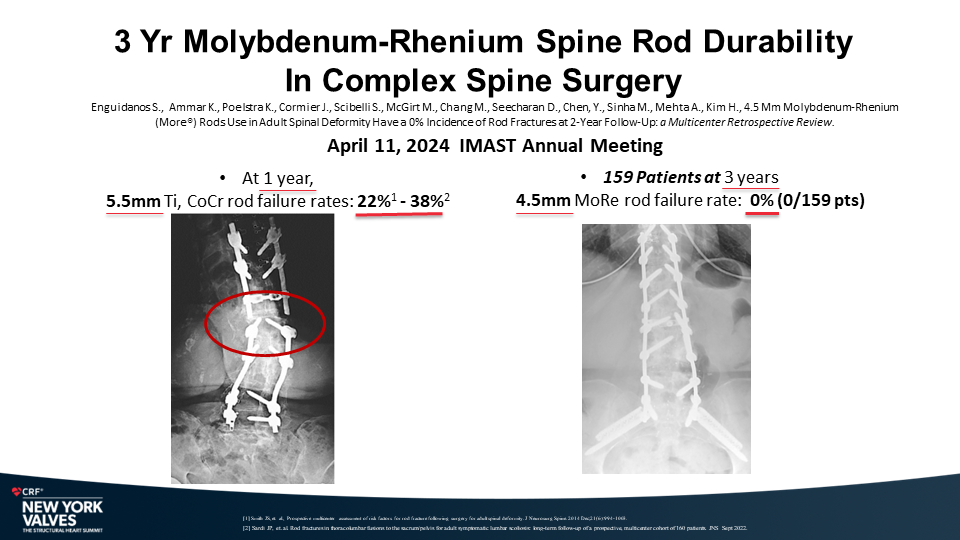
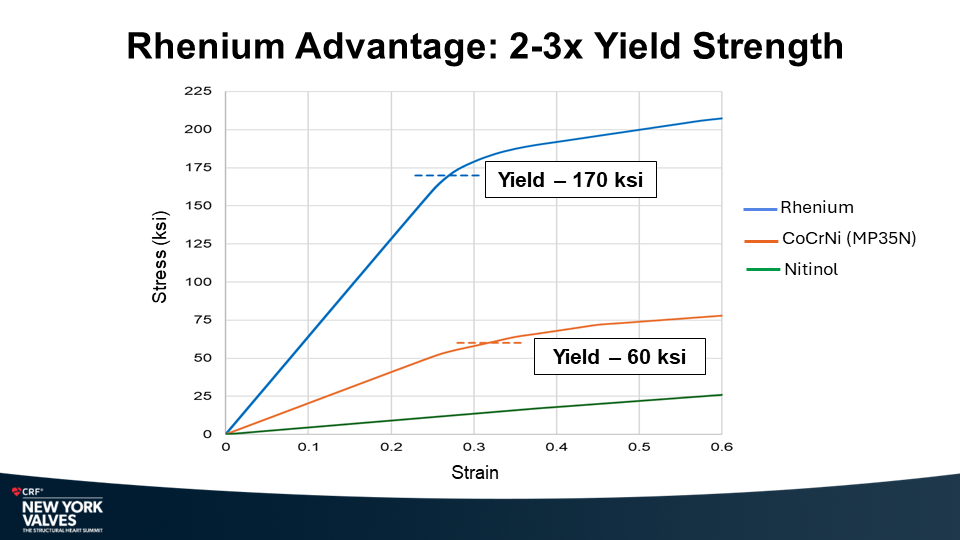
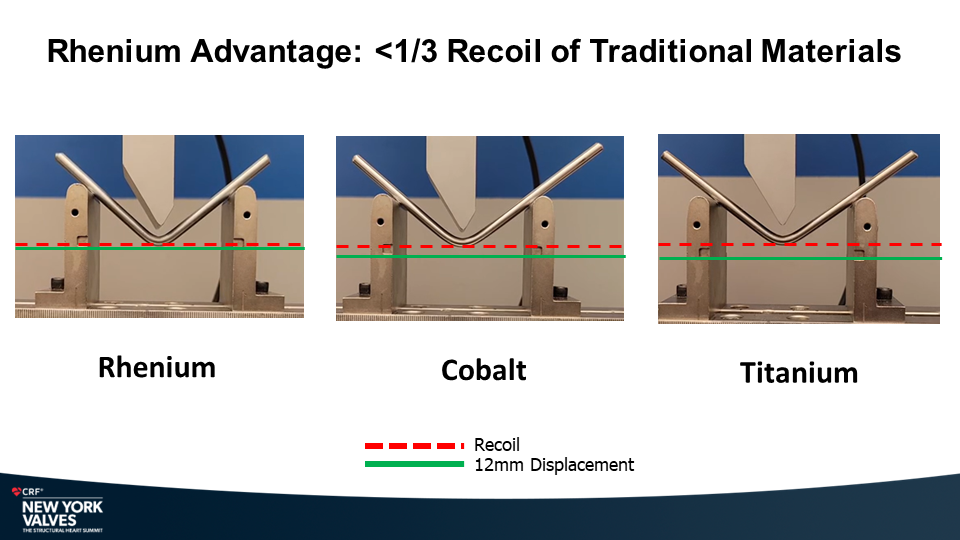
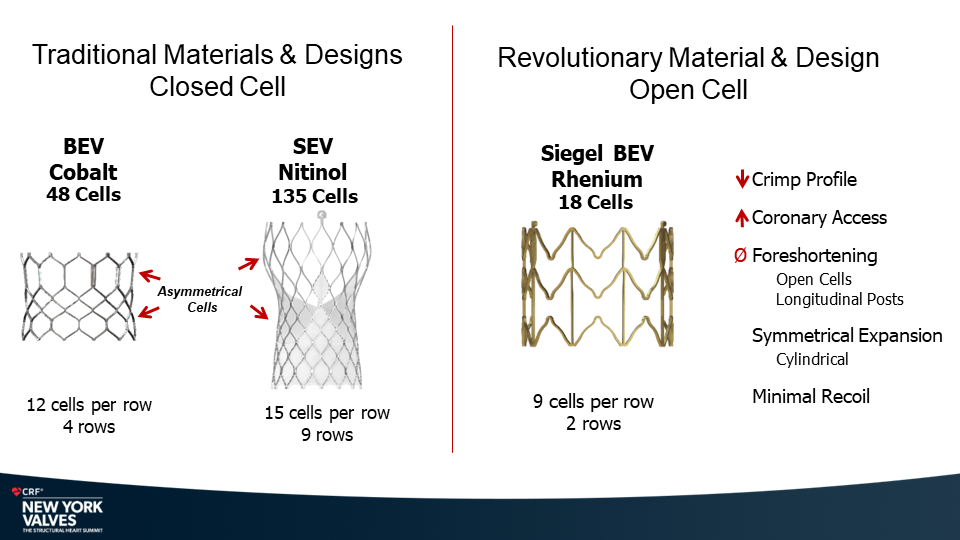
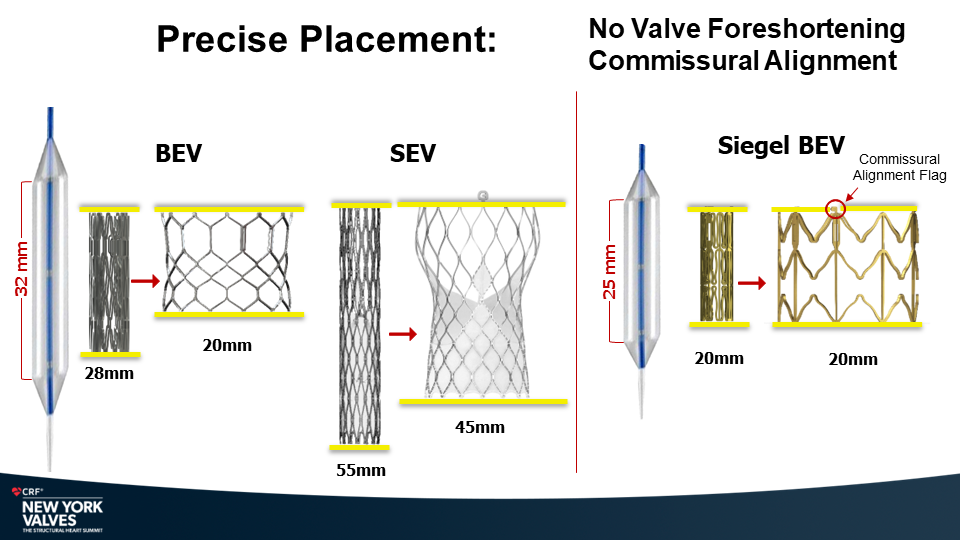
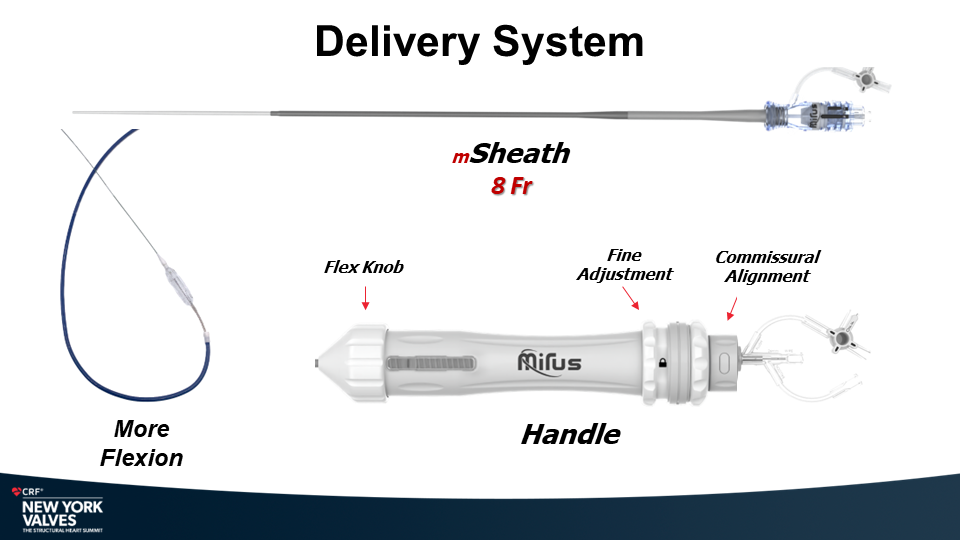

Molybdenum-rhenium(MoRe®) rods in complex adult spine surgery without rod fractures: a multicenter retrospective case series.
Enguidanos S., et.al
Congress of Neurological Surgeons (CNS) Annual Meeting.
September 13, 2023; Washington DC.
Introduction:
In complex spine surgery, current pedicle screw systems with 5.5mm titanium, cobalt chromium or stainless steel rods have rod failure rates of approximately 9% with a minimum of 1-year follow-up. The fracture rate with these alloys increases to approximately 22% in pedicle subtraction osteotomy cases1. Rod fracture have significant morbidity for patients, including pain, loss of deformity correction, and the need for revision surgery. In laboratory testing, molybdenum-rhenium(MoRe) has superior yield strength and fatigue endurance limit compared to Ti-6Al-4V and CoCr.
Objective:
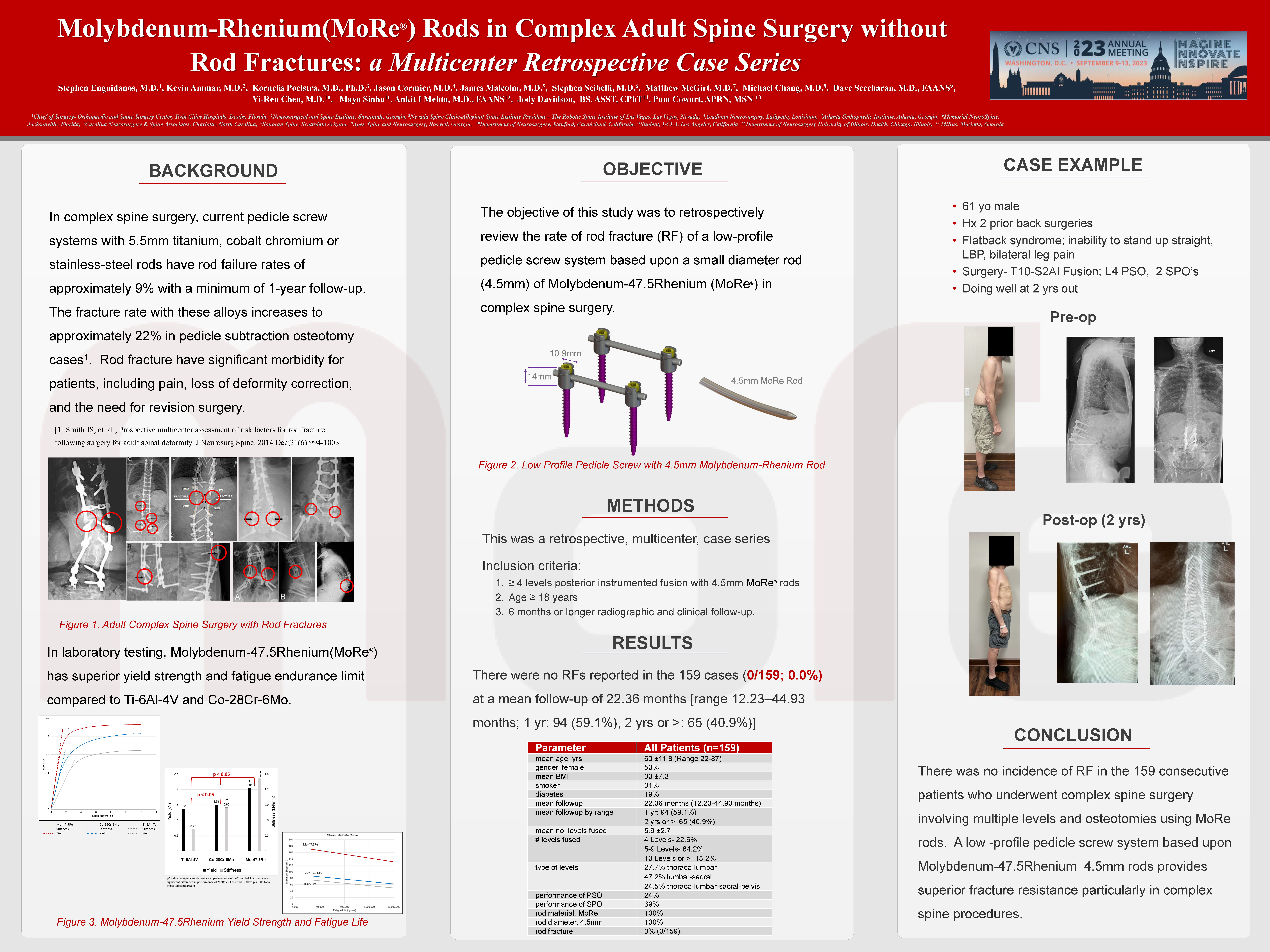
The objective of this study was to retrospectively review the rate of rod fracture (RF) of a low profile pedicle screw system based upon a small diameter rod (4.5mm) of molybdenum-rhenium (MoRe) in complex spine surgery.
Hypothesis:
A 4.5mm molybdenum-rhenium(MoRe) rod will have adequate performance and durability in complex spine surgeries.
Methods:
This was a retrospective, multicenter, case series. Inclusion criteria were ≥ 4 levels posterior instrumented fusion with 4.5mm MoRe rods, age ≥ 18 years and 6 months or longer radiographic and clinical follow-up.
Results:
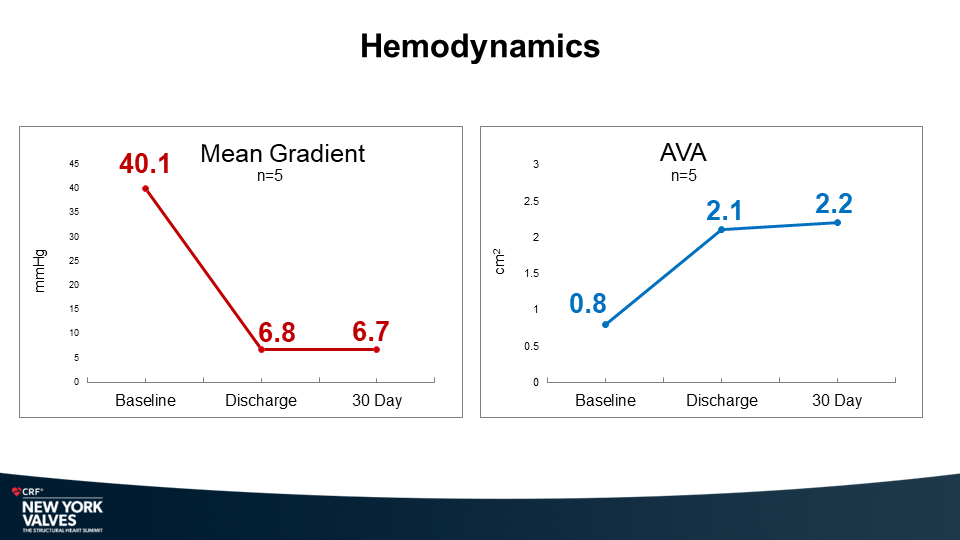
One hundred and fifty-nine (159) consecutive patients from ten (10) different medical centers that had spinal surgery from August 2019 until April 2022 met the inclusion criteria. The patients’ mean age was 63 ±11.8 years; 50% were women; 31% were smokers; 19% were diabetic and the mean body mass index (BMI) was 30 ±7.3. The mean number of levels fused was 5.9 ±2.7; 22.6% were 4 levels, 64.2% were 5-9 levels and 13.2% were 10 levels or greater. Approximately half were thoracolumbar or thoracolumbar to pelvis (27.7% thoracolumbar, 24.5% thoracolumbar to pelvis) and approximately half (47.2%) were lumbar-sacral. Thirty-eight (38) patients (24%) had a pedicle subtraction osteotomy (PSO) and 59 patients (39%) had a Smith Peterson Osteotomy (SPO). All cases were done only with molybdenum-rhenium 4.5mm diameter rods. There were no RFs reported in the 159 cases (0/159; 0.0%) at a mean follow-up of 22.36 months .
Conclusion:
There was no incidence of RF in the 159 consecutive patients who underwent complex spine surgery involving multiple levels and osteotomies using MoRe rods. A low profile pedicle screw system based upon molybdenum-rhenium (MoRe) 4.5mm rods provides superior fracture resistance particularly in complex spine procedures.
Smith JS, et. al., Prospective multicenter assessment of risk factors for rod fracture following surgery for adult spinal deformity. J Neurosurg Spine. 2014 Dec;21(6):994-1003.
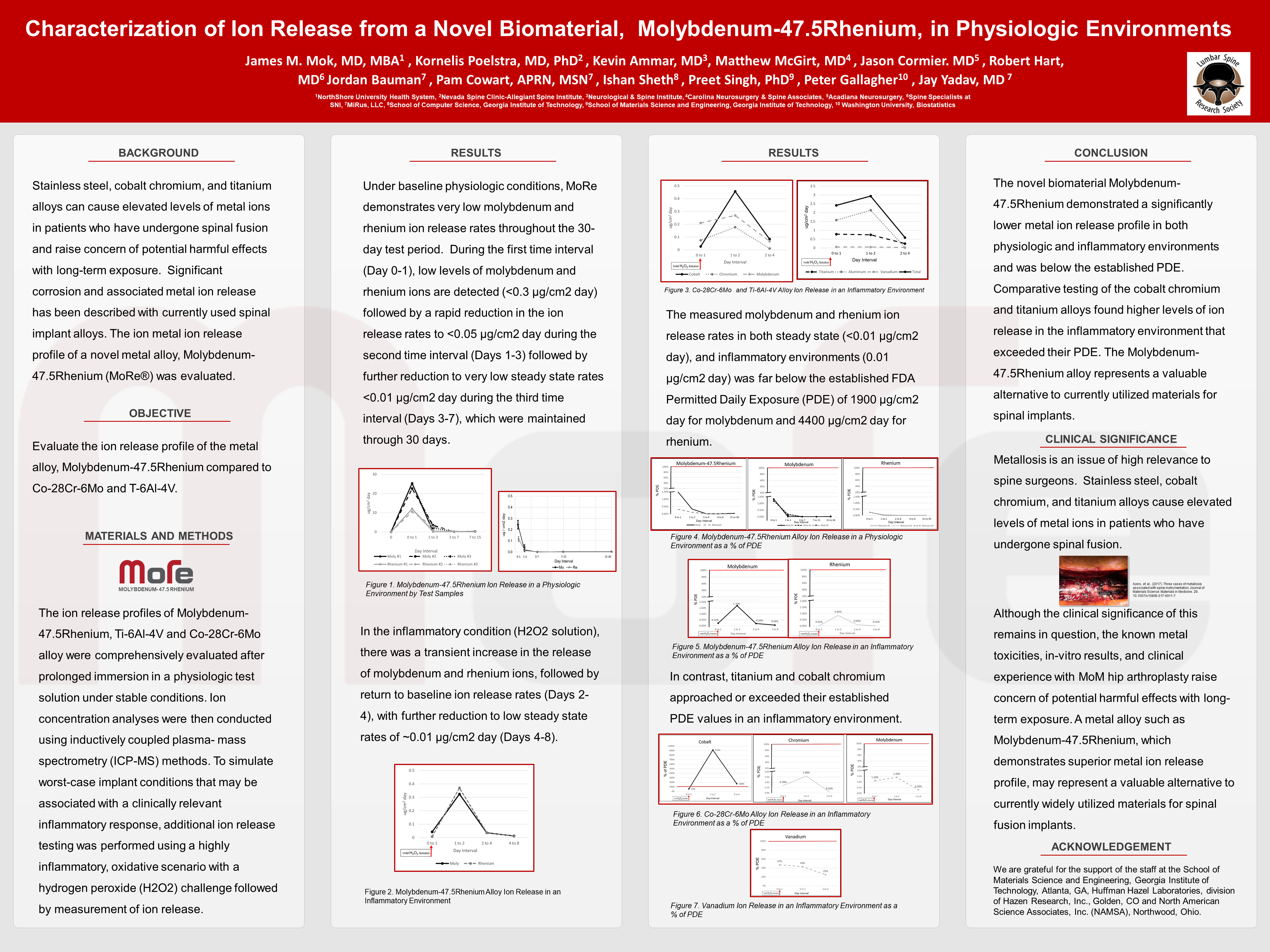
Characterization of Ion Release from a Novel Biomaterial, Molybdenum-47.5Rhenium, in Physiologic Environments
Mok J., et.al
Lumbar Spine Research Society (LSRS) 16th Annual Meeting.
May 11, 2023; Chicago, Illinois.
Introduction:
Metals from spinal implants are released into surrounding tissues by various mechanisms. Metal ion release has been associated with clinical implant failure, osteolysis, and remote site accumulation with adverse events. Significant corrosion and associated metal ion release has been described with currently used spinal implant alloys. A novel metal alloy, Molybdenum-47.5Rhenium Alloy (MoRe), was approved for use in medical implants in 2019 by the FDA.
Purpose:
To evaluate the metal ion release profile of Molybdenum-47.5Rhenium alloy after immersion in both a stable physiologic, as well as in an inflammatory environment
Methods:
The ion release profile of MoRe alloy was comprehensively evaluated in-vitro after prolonged immersion in physiologic and inflammatory environments. Ion concentration analyses were then conducted using inductively coupled plasma - mass spectrometry (ICP-MS) methods. Comparative testing of titanium (Ti-6Al-4V) and cobalt chromium (Co-28Cr-6Mo) was also performed.
Results:
Under baseline physiologic conditions, the MoRe alloy demonstrates very low molybdenum and rhenium ion release rates throughout the 30-day test period. During the first time interval (Day 0 to 1), low levels of molybdenum and rhenium ions are detected (<0.3 μg/cm2 day) followed by a rapid reduction in the ion release rates to <0.05 μg/cm2 day during the second time interval (Days 1 to 3) followed by further reduction to very low steady state rates <0.01 μg/cm2 day during the third time interval (Days 3 to 7), which were maintained through 30 days. In the inflammatory condition (H2O2 solution), there was a transient increase in the release of molybdenum and rhenium ions, followed by return to baseline ion release rates (Days 2 to 4), with further reduction to low steady state rates of ~0.01 μg/cm2 day (Days 4 to 8). The measured molybdenum and rhenium ion release rates in both steady state (<0.01 μg/cm2 day), and inflammatory environments (0.01 μg/cm2 day) was far below the established FDA Permitted Daily Exposure (PDE) of 1900 μg/cm2 day for molybdenum and 4400 μg/cm2 day for rhenium. In contrast, titanium and cobalt chromium approached or exceeded their established PDE values in an inflammatory environment.
Conclusions:
The novel biomaterial Molybdenum-47.5Rhenium demonstrated a lower metal ion release profile in both a physiologic and inflammatory environment and was well below the established PDE. Comparative testing of the cobalt chromium and titanium alloys found higher levels of ion release in the inflammatory environment that exceeded the PDE for cobalt and vanadium.
Clinical Significance
Stainless steel, cobalt chromium, and titanium alloys cause elevated levels of metal ions in patients who have undergone spinal fusion. The known metal toxicities, in-vitro results, and clinical experience with metal-on-metal hip arthroplasty raise concern of potential harmful effects with long-term exposure. A metal alloy such as Molybdenum-47.5Rhenium, which demonstrates a superior metal ion release profile, may represent a valuable alternative to currently widely utilized materials for spinal fusion implants.
Characterization of Ion Release from a Novel Biomaterial, Molybdenum-47.5Rhenium, in Physiologic Environments
Mok J., Poelstra K., Ammar K., McGirt M., Cormier J., Hart R., Bauman J., Cowart P., Sheth I., Singh P. and Yadav J.
The Spine Journal. January 24, 2023; https://doi.org/10.1016/j.spinee.2023.01.007.
Introduction:
Metals from spinal implants are released into surrounding tissues by various mechanisms. Metal ion release has been associated with clinical implant failure, osteolysis, and remote site accumulation with adverse events. Significant corrosion and associated metal ion release has been described with currently used spinal implant alloys. A novel metal alloy, Molybdenum-47.5Rhenium Alloy (MoRe), was approved for use in medical implants in 2019 by the FDA.
Purpose:
To evaluate the metal ion release profile of Molybdenum-47.5Rhenium alloy after immersion in both a stable physiologic, as well as in an inflammatory environment
Methods:
The ion release profile of MoRe alloy was comprehensively evaluated in-vitro after prolonged immersion in physiologic and inflammatory environments. Ion concentration analyses were then conducted using inductively coupled plasma - mass spectrometry (ICP-MS) methods. Comparative testing of titanium (Ti-6Al-4V) and cobalt chromium (Co-28Cr-6Mo) was also performed.
Results:
Under baseline physiologic conditions, the MoRe alloy demonstrates very low molybdenum and rhenium ion release rates throughout the 30-day test period. During the first time interval (Day 0 to 1), low levels of molybdenum and rhenium ions are detected (<0.3 μg/cm2 day) followed by a rapid reduction in the ion release rates to <0.05 μg/cm2 day during the second time interval (Days 1 to 3) followed by further reduction to very low steady state rates <0.01 μg/cm2 day during the third time interval (Days 3 to 7), which were maintained through 30 days. In the inflammatory condition (H2O2 solution), there was a transient increase in the release of molybdenum and rhenium ions, followed by return to baseline ion release rates (Days 2 to 4), with further reduction to low steady state rates of ~0.01 μg/cm2 day (Days 4 to 8). The measured molybdenum and rhenium ion release rates in both steady state (<0.01 μg/cm2 day), and inflammatory environments (0.01 μg/cm2 day) was far below the established FDA Permitted Daily Exposure (PDE) of 1900 μg/cm2 day for molybdenum and 4400 μg/cm2 day for rhenium. In contrast, titanium and cobalt chromium approached or exceeded their established PDE values in an inflammatory environment.
Conclusions:
The novel biomaterial Molybdenum-47.5Rhenium demonstrated a lower metal ion release profile in both a physiologic and inflammatory environment and was well below the established PDE. Comparative testing of the cobalt chromium and titanium alloys found higher levels of ion release in the inflammatory environment that exceeded the PDE for cobalt and vanadium.
Clinical Significance
Stainless steel, cobalt chromium, and titanium alloys cause elevated levels of metal ions in patients who have undergone spinal fusion. The known metal toxicities, in-vitro results, and clinical experience with metal-on-metal hip arthroplasty raise concern of potential harmful effects with long-term exposure. A metal alloy such as Molybdenum-47.5Rhenium, which demonstrates a superior metal ion release profile, may represent a valuable alternative to currently widely utilized materials for spinal fusion implants.
Low Profile 4.5mm Molybdenum-Rhenium System for MIS Single Position Prone Lateral Spine Surgery
Cormier J.
AANS/CNS Joint Section Spine Summit 2023 Annual Meeting;
March 16-19, 2023
Introduction:
Minimally invasive anterolateral approaches to the lumbar spine are options for the treatment of a number of adult degenerative spinal disorders. Dissection and dilation through the iliopsoas muscle places the lumbosacral plexus at risk for injury and potential devastating complications. A new technique of MIS single position prone lateral surgery using a low profile system composed of smaller pedicle screws, 4.5mm molybdenum-rhenium rods and specialized lateral retractor can reduce the frequency of complications associated with lateral approaches. .
Methods:
This was a retrospective, single center, case series. Inclusion criteria were adult (≥ 18 ) patients who underwent a prone lateral MIS LIF with the low profile molybdenum-rhenium system. Intraoperative and postoperative adverse events immediately after surgery and during routine follow-up intervals were examined.
Results:
Twenty-four (24) patients that had spinal surgery from March 2020 until November 2022 met the inclusion criteria. The patients’ mean age was 64 ±8.9 years; 37.5% were women; 41.7% were smokers; 4.2% were diabetic with a mean body mass index (BMI) of 31.6 ±7.8.0. The mean number of levels fused was 1.7 ±1.2 with an average EBL of 123.9 cc. There were no occurrences of postoperative ipsilateral thigh numbness or weakness.
Conclusions:
The MIS single position prone lateral approach is a safe alternative to traditional open, direct lateral and extreme lateral operations for many spinal disorders. Our refined technique of MIS Prone Lateral using a low profile molybdenum-rhenium system resulted in excellent safety without occurrence of postoperative ipsilateral thigh numbness, ipsilateral iliopsoas or quadriceps weakness.
Biological Fixation of 3DR™ Randomized Printed Implants in a Vertebral Defect Ovine Model
Mok J , et. al.
North American Spine Society (NASS) Annual Meeting
September 30, 2021
Background: The most commonly used materials used for interbody cages are titanium alloys and polymer polyetheretherketone (PEEK) both of which have demonstrated good biocompatibility but have major disadvantages. A solid titanium cage is radiopaque which limits the postoperative monitoring of spinal fusion via standard imaging modalities and it also has a high modulus of elasticity which has been postulated to lead to increased rates of subsidence. PEEK is radiolucent yet it is hydrophobic, which can inhibit bony growth into the cages from the vertebral end plates, resulting in pseudarthrosis.
Innovations are being developed using additive manufacturing approaches which can enhance the biomechanical properties of a structural cage. A 3DR™ randomized printed titanium (3DR™ Ti) implant, has been designed with a randomized lattice structure that mimics the organic structure of cancellous bone, providing a favorable environment to potentiate better bone apposition and ingrowth while enabling imaging of the graft window within the interbody device.
Purpose: The study objective was to examine the healing response of a 3DR™ Ti implant with a randomized lattice structure in comparison to a control device of polyetheretherketone (PEEK) in an in vivo vertebral defect ovine model.
Methods: Two defects were created within each vertebra (L2-L5) via a left lateral retroperitoneal approach in ten skeletally mature sheep (n = 10). 6 x 10 mm long cylindrical implants of either smooth PEEK or 3DR™ Ti were press-fit into each vertebral defect. Five animals were sacrificed per timepoint post-surgery (6, 12 weeks) allotting 5 and 5 samples for histomorphometry and biomechanics, respectively, per group per time point. Biomechanical and histomorphometry analyses were conducted on samples to evaluate the osseointegration performance of the 3DR™ Ti and PEEK implants.
Results: No implant related complications were noted in any animals throughout the study. Statistically significant differences were observed in the biomechanical pushout force and shear strength (increase ultimate load , increase ultimate stress, increase ultimate displacement) with the 3DR™ printed Ti implants compared to the PEEK implant at both 6 weeks (P<0.001) and 12 weeks (P<0.001) timepoints and across the 6 to 12 week timepoint. (P<0.001) Histomorphometry data showed statistically significant improvement in osseointegration associated with the 3DR™ Ti implant exhibited by significantly increased percent bone area as compared to the PEEK implant at 6 weeks (P=0.018) and significant increase in the percent bone area from the 6-week timepoint to the 12-week timepoint in the 3DR™ Ti implant. (P=0.020)
Conclusions: The 3DR™ Ti implant’s randomized lattice structure had a significant effect on biological fixation in sheep vertebrae as shown through statistically significant increases in the biomechanical pushout force, shear stress, stiffness, bone-implant contact and bony fill when compared to a PEEK implant. The 3DR™ Ti implant provides a superior environment to potentiate better bone apposition and ingrowth when compared to PEEK implant.
3DR™ Printed Lumbar Interbody Fusion System provides an Advanced Option for Circumferential Fusion of the Lumbar Spine
Mok J, et al.
North American Spine Society
October 2020
Background Context: Circumferential fusion of the lumbar spine has been associated with improved clinical results and durability of the outcomes compared with posterolateral fusion. Interbody cages are useful in circumferential fusion to improve segmental stability, alignment of the spine, and interbody arthrodesis. Although interbody cages have been routinely used to achieve fusion for over a decade, existing designs fall short of providing all the necessary characteristics for clinical success.
The most commonly used materials used for interbody cages are titanium alloys and polymer polyetheretherketone (PEEK) both of which have demonstrated good biocompatibility but have major disadvantages. A solid titanium cage is radiopaque which limits the postoperative monitoring of spinal fusion via standard imaging modalities and it also has a high modulus of elasticity which has been postulated to lead to increased rates of subsidence. PEEK is radiolucent yet it is hydrophobic, which can inhibit bony growth into the cages from the vertebral end plates, resulting in pseudarthrosis.
Although both PEEK and titanium cages have been successful at achieving spinal fusion, innovations are being developed using additive manufacturing approaches which can enhance the biomechanical properties of a structural cage. The 3DR™ Lumbar Interbody device, has been designed with a randomized lattice structure that mimics the organic structure of cancellous bone, providing a favorable environment to potentiate better bone apposition and ingrowth while enabling imaging of the graft window within the interbody device.
Purpose: To evaluate the stiffness and porosity of the 3DR™ Lumbar Interbody System in comparison to currently marketed 3D printed interbody devices.
Methods: Multiple tests were performed to evaluate the elastic modulus and porosity properties of the 3DR™ Lumbar Interbody System.
- Elastic Modulus Evaluation
A quantity of three 3DR™ ALIFs were tested in the elastic region under axial compression. The force and displacement values were then converted into stress and strain and plotted. The slope of the stress strain curve in the elastic region is defined as the modulus of elasticity. The slopes are in Megapascals and were converted to Gigapascals. The average of the three modulus values was then taken.
- Effective Density
The total mass of two comparator 3D interbodies and three 3DR interbodies ( large LLIF, small LLIF and ALIF) were calculated in grams. The total mass was then divided by the total volume encompassed by each interbody in cm^3 to derive the effective density value.
Results: The 3DR lumbar Interbody system has an elastic modulus of 0.53 GPa within the range of cancellous bone at 0.5 GPa and significantly lower than cortical bone (12-18 GPa) or solid titanium (101-110 GPa). Current traditional 3D printed interbodies on today’s market report an elastic modulus in the range of 0.9 to 6.2 GPa well above cancellous bone.
The 3DR lumbar interbody system including the small LLIF, large LLIF and ALIF have an effective density significantly less (1.14 g/cm˄3, 0.97 g/cm˄3, 0.76 g/cm˄3 respectively) than current traditional 3D printed interbodies on the market.
Conclusions: The 3DR™ Interbody System with a stiffness of 0.53 GPa, has the lowest stiffness of any traditional 3D printed interbody on the market and is in the same range as cancellous bone. Due to their thin walls and randomized lattice structure, the 3DR interbodies occupy the same volume using less metal leading to more radiolucency than other interbodies on the market.
Validation of a Wireless, Non-Optical System for Measurement of Intra-Operative Spine Alignment
Nottmeier E, et al.
AANS/CNS Spine Joint Section Meeting
March 2019
Introduction: Sagittal alignment of the spine is closely related to quality of life scores and malalignment is a cause of pain and disability. Current methods of intra-operative measurements are inadequate for real-time measurement of segmental and overall spinal alignment. The objective of this study was to validate a wireless, non-optical system for dynamic assessment of spine alignment during surgery.
Methods: The intra-operative spine alignment monitoring system (SAM) utilizes disposable, miniaturized wireless MEMS sensors, advanced signal processing, and error compensation algorithms to provide dynamic measurement of sagittal plane intervertebral angles and overall spinal alignment. The sensing module is battery-powered and communicates information wirelessly to the display unit. Registration is performed with a single fluoroscopic image. The goal is to provide continuous tracking of spine alignment without reliance on repeated imaging.
System accuracy was validated via benchtop and simulated use-testing. Benchtop testing simulated motions that could be encountered during surgery using precision stages that allowed for accurate reference values for angular measurements. Simulated clinical use-testing using radiopaque Sawbones® phantoms compared manual Cobb angle measurements of Lordosis and Kyphosis on lateral fluoroscopic images to the values measured by the SAM.
Results: In benchtop testing, mean difference between the reference values and the spine alignment monitoring system measurements was 0.0 degrees with a standard deviation of 0.9 degrees (n= 90). In simulated use testing, mean difference between the cobb angle measurements and the spine alignment monitoring system measurements was 1.5 degrees with a standard deviation of 1.9 degrees (n = 30).
Conclusion: Benchtop and simulated use testing of the SAM demonstrated high accuracy and repeatability. This approach could provide surgeons with practical, real-time measurements of segmental and overall alignment, thus allowing attainment of sagittal balance.
Validation of a Wireless, Non-Optical System for Measurement of Intra-Operative Spine Alignment
Daftari T, et al.
Southern Orthopaedic Society Annual Meeting
July 2019
Introduction: Sagittal alignment of the spine is closely related to quality of life scores and malalignment is a cause of pain and disability. Current methods of intra-operative measurements are inadequate for real-time measurement of segmental and overall spinal alignment. The objective of this study was to validate a wireless, non-optical system for dynamic assessment of spine alignment during surgery.
Methods: The intra-operative spine alignment monitoring system (SAM) utilizes disposable, miniaturized wireless MEMS sensors, advanced signal processing, and error compensation algorithms to provide dynamic measurement of sagittal plane intervertebral angles and overall spinal alignment. The sensing module is battery-powered and communicates information wirelessly to the display unit. Registration is performed with a single fluoroscopic image. The goal is to provide continuous tracking of spine alignment without reliance on repeated imaging.
System accuracy was validated via benchtop and simulated use-testing. Benchtop testing simulated motions that could be encountered during surgery using precision stages that allowed for accurate reference values for angular measurements. Simulated clinical use-testing using radiopaque Sawbones® phantoms compared manual Cobb angle measurements of Lordosis and Kyphosis on lateral fluoroscopic images to the values measured by the SAM.
Results: In benchtop testing, mean difference between the reference values and the spine alignment monitoring system measurements was 0.0 degrees with a standard deviation of 0.9 degrees (n= 90). In simulated use testing, mean difference between the cobb angle measurements and the spine alignment monitoring system measurements was 1.5 degrees with a standard deviation of 1.9 degrees (n = 30).
Conclusion: Benchtop and simulated use testing of the SAM demonstrated high accuracy and repeatability. This approach could provide surgeons with practical, real-time measurements of segmental and overall alignment, thus allowing attainment of sagittal balance.
Semi-automated Method for Calculation of Sagittal Alignment from CT Scans
Nottmeier E, et al.
AANS/CNS Spine Joint Section Meeting
March 2019
Background: Sagittal alignment of the spine is closely related to quality of life scores and malalignment is a cause of pain and disability. Stabilization of the spine and restoration of normal posture and spine alignment using osteotomies and/or fusion is a major goal of spine surgery.
Current manual methods of measurement of sagittal alignment (Cobb, etc) on 2D sagittal radiographs have high inter and intra observer variability and are considered too time-consuming and complex for routine clinical use. Computerized measurements of sagittal alignment can reduce human variability and enable objective and consistent interpretation of images.
Purpose: To validate a semi-automated method for calculation of sagittal plane alignment from CT scans.
Methods: A semi-automated method for computerized calculation of sagittal plane alignment was developed. The method is based on processing and analyzing a CT scan of the spine. The CT scan is first segmented into individual vertebral bodies using a semi-automated algorithm with minimal human intervention. After segmentation, the algorithm analyzes the spatial relationship between the segmented vertebral bodies and calculates the spinal curvature and intervertebral alignment/angles.
Lumbar Lordosis (L1-S1 angle) was calculated on the deidentified CT scans of 9 patients using two methods 1) Manual Cobb method and 2) Semi-automated computerized method. The mean difference, standard deviation, and coefficient and determination (R2) values were calculated.
Results: The mean difference in Lordosis calculated using the manual and computerized methods was 0.1 degrees with a standard deviation on 3.8 degrees (n=9). The R2 value was 0.9526.
Molybdenum-Rhenium (MoRe®) Alloy and Novel Sensor Technology allows for a Mechanically and Biologically Superior Option for Spinal Implants and Remote Real-Time Monitoring of Bone Growth Across a Fixated Motion Segment
Poelstra K, et al.
EOA Annual Meeting
October 2018
Background: Ti-6Al-4V-ELI (Ti-ELI) and Cobalt Chromium (CoCr), alloys used for spinal implants, have limited strength and fatigue resistance therefore requiring a larger implant size. Our aim is to develop a rod construct alternative made of MoRe® that can leverage its superior mechanical properties to reduce rod size and incorporate dynamic sensor technology.
Methods: Three individual experiments were conducted:
1: ASTM-1717 test methods for spinal implant constructs in a vertebrectomy model to evaluate mechanical performance of 4.0mm diameter MoRe® alloy vs. 5.5mm diameter Ti-ELI and CoCr rods;
2: Bone implantation study to evaluate the local tissue and bone response of MoRe® vs. Ti-ELI when implanted in the femoral bone of rabbits for 4, 13 and 26 weeks;
3: In-vivo experiment to evaluate mechanical stabilization of a tibial defect with a novel SMART™ sensor incorporated implant measuring micro-strain correlated with imaging evidence of bone growth in a sheep fracture model over 6 weeks.
Results:
The three individual experiments yielded the following results:
1: Reduced diameter MoRe® (4.0mm), demonstrated superior mechanical performance versus 5.5mm diameter Ti-ELI and CoCr:
2: MoRe® and Ti-ELI implant sites demonstrated similar osteoconduction and bone remodeling histology of cortical and medullary bone at all time points.
3: Implant micro-strain of 901µε at time of surgery decreased to 132.5µε at 4 weeks and 31.8µε at 6 weeks post-implant correlating (p<0.05) with radiographic appearance of partial and complete fracture healing.
Conclusion: MoRe® implants, with embedded novel sensor technology allow for a new generation of smaller, more fatigue resistant, bio-friendly SMART™ implants that may be able to detect evolving pseudoarthrosis prior to clinical presentation.
Molybdenum-Rhenium (MoRe®) Alloy and Novel Sensor Technology Allows for a Mechanically and Biologically Superior Option for Spinal Implants and Remote Real-time Monitoring of Bone Growth Across a Fixated Motion Segment
McGirt M, et al.
CNS Annual Meeting
October 2018
Background: Ti-6Al-4V-ELI (Ti-ELI) and Cobalt Chromium (CoCr), alloys used for spinal implants, have limited strength and fatigue resistance therefore requiring a larger implant size. Our aim is to develop a rod construct alternative made of MoRe® that can leverage its superior mechanical properties to reduce rod size and incorporate dynamic sensor technology.
Methods: Experiments conducted: ASTM-1717 test methods for spinal implant constructs in a vertebrectomy model to evaluate mechanical performance of 4.0mm diameter MoRe® alloy vs. 5.5mm diameter Ti-ELI and CoCr rods; bone implantation study to evaluate the local tissue and bone response of MoRe® vs. Ti-ELI when implanted in the femoral bone of rabbits for 4, 13 and 26 weeks; in-vivo experiment to evaluate mechanical stabilization of a tibial defect with a novel sensor incorporated implant measuring micro-strain correlated with imaging evidence of bone growth in a sheep over 6 weeks.
Results:
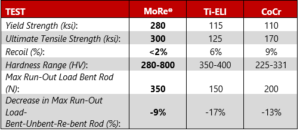
Reduced diameter MoRe® (4.0mm), demonstrated superior mechanical performance versus 5.5mm diameter Ti-ELI and CoCr:
Yield Strength (ksi): MoRe® 280 ksi; Ti-ELI 115 ksi; CoCr 110 ksi
Ultimate Tensile Strength (ksi): MoRe® 300 ksi; Ti-ELI 125 ksi; CoCr 170 ksi
Recoil (%): MoRe® <2%; Ti-ELI 6%; CoCr 9%
Hardness Range (HV): MoRe® 280-800 HV; Ti-ELI 350-400 HV; CoCr 255-331 HV
Max Run-Out Load Bent Rod (N): MoRe® 350 N; Ti-ELI 150 N; CoCr 200 N
Decrease in Max Run-Out Load-
Bent-Unbent-Re-bent Rod (%): MoRe® 9%; Ti-ELI 17%; CoCr 13%
MoRe® and Ti-ELI implant sites demonstrated similar osteoconduction and bone remodeling histology of cortical and medullary bone at all time points.
Implant micro-strain of 901µε at time of surgery decreased to 132.5µε at 4 weeks and 31.8µε at 6 weeks post-implant correlating (p<0.05) with radiographic appearance of partial and complete fracture healing.
Molybdenum Rhenium (MoRe®) as a Biologically Superior Alloy for Foot and Ankle Implants
Pedowitz, D. et. al.
AOFAS Annual Meeting
July 2018
Introduction: The most common complications in orthopaedic surgery of the foot and ankle documented in the literature are postoperative infections with a frequency between 1 and 8%. The development of an implant-associated infection can be a potentially devastating complication following foot and ankle surgery. This leads to a high number of revision surgeries after initial fracture stabilization and potential loss of function of the joint and quality of life in the long-term follow-up.
Titanium, the most commonly used alloy for foot and ankle implants, has limited strength and is notch-sensitive so repetitive stress leads to fatigue failure of implants and limits design options. Better materials with both optimized mechanical and biological properties could result in the development of superior foot and ankle implants.
Purpose: To evaluate the biological properties of Molybdenum-Rhenium (MoRe®) for foot and ankle implants.
Methods: Several studies were performed to evaluate the biological properties of MoRe®:
- A study was conducted to characterize the hydrophilic properties by contact angle comparisons of the MoRe® alloy compared to Titanium.
- A cell growth study was conducted to look at the influence of MoRe® alloy on osteoblast growth and differentiation as compared to Titanium.
- A bone implantation study was conducted based on ISO 10993: Biological Evaluation of Medical Devices, Part 6: Tests for Local Effects after Implantation to evaluate the local tissue and bone response of MoRe® alloy test coupon compared to Titanium controls, when implanted in the mid-shaft femoral bone of rabbits for 4, 13 and 26 weeks.
- Biofilm formation study was conducted to quantify attachment and biofilm development of S. aureus bacteria upon MoRe® alloy in comparison CoCr alloy in a well-controlled, reproducible, ex vivo laboratory experiment.
Results: MoRe®, composed purely (99.99%) of molybdenum and rhenium, is a cofactor to the enzymes xanthine and sulfite oxidase, which are essential to bone metabolism. Rhenium is an inert metal with no biological affect.
MoRe® has superior hydrophilicity (CA 37°±3°) compared to Titanium (CA 58°±3°), a key factor in cell adhesion, migration and replication.
Pre-osteoblast cell seeding was found to be equivalent between MoRe® and Titanium alloy (10,000 cells/cm2).
The bone implantation study demonstrated continued bone maturation over time for both MoRe® and Titanium implant sites with similar osteoconduction and bone remodeling histology of the cortical and medullary bone at 4, 13 and 26 weeks.
The biofilm study found that S. aureus better colonized cobalt chrome alloy in comparison to MoRe® (p = 0.009).
Conclusions: The MoRe® alloy, with its advantageous biological properties and hydrophilicity, offers great promise for the design of a new generation of bio-friendly foot and ankle implants with superior cell growth, osseointegration, and biofilm resistance resulting in better outcomes for patients.
1Olsen LL, et al. The impact of lifestyle risk factors on the rate of infection after surgery for a fracture of the ankle. Bone Joint J. 2017;99-B(2):225–30
2Tome-Bermejo F, Santacruz Arevalo A, Ruiz Mico N. Open reduction and internal fixation of displaced ankle fractures in patients older than 65 years of age. Analysis of results at five-year follow-up. Rev Esp Cir Ortop Traumatol. 2016;60(2):99–105.
3Zalavras CG, et al. Infection following operative treatment of ankle fractures. Clin Orthop Relat Res. 2009;467(7):1715–20.
Metallurgical Properties of MO-47.5RE Implant Alloy
Disegi J.
Materials and Surface Technology for Implants: Meet the Expert (Implants), Olten, Switzerland
March 13, 2018
Introduction: Wrought MoRe® powder metallurgy (PM) alloys were originally developed for high temperature heat treating and aerospace components. The Nuloy coronary stent from Icon Interventional was clinically evaluated per CE certification 574558. A Mo-47.5Re (MoRe®) alloy composition was selected to investigate potential orthopedic applications.
Methods: Composition, physical, tensile and biocompatibility properties are compiled in ASTM F3273-17 standard . Dynamic fatigue properties in air were compared for MoRe®, CoCr, and TAV ELI bent spine rods according to ASTM F1717 except runout load was documented at 2.5 million (M) rather than 5 M cycles. Bone implantation testing per ISO 10993-6 compared 1.5 mm Ø X 6 mm long MoRe® and TAV ELI pins in rabbit mid-shaft femurs containing a cortical defect.
Results: Mo-47.5Re alloy limits in Table 1 include fourteen interstitial and residual elements which define a very pure binary implant alloy.
|
Table 1. Mo-47.5Re composition limits. |
|
|
Element |
Weight % |
|
N, H, Fe, O, S, Ti, Si |
max 0.010 |
|
Mn, P, Cu, B, Sn |
max 0.010 |
|
C,W |
max 0.050 |
|
Re |
46.0-49.0 |
|
Mo |
balance |
MoRe® alloy has the highest density (13.52 gm/cm3) and highest modulus of elasticity (365 GPa) when compared to contemporary titanium-base, cobalt-base, and stainless steel implant materials. Mo-47.5Re alloy has a lower magnetic susceptibility than commercially pure (CP) titanium and accounts for the reduced amount of magnetic resonance imaging (MRI) artifact. Minimum tensile properties are shown in Table 2.
|
Table 2. Minimum tensile properties for cold worked (CW) and extra hard (EH) bar. |
||
|
CW |
EH |
|
|
UTS (MPa) |
1240 |
1380 |
|
0.2%YS (MPa) |
1100 |
1310 |
|
Elong (%) |
12 |
10 |
|
ROA (%) |
40 |
45 |
Fatigue properties are highlighted in Table 3.
|
Table 3. Runout load @ 2.5M cycles for bent spine rods. |
|||
|
Diam (mm) |
MoRe® (N) |
CoCr (N) |
TAV ELI (N) |
|
4.0 |
350 |
--- |
--- |
|
5.5 |
--- |
200 |
150 |
Discussion: Cold working provides high strength and high ductility due to twinning induced plasticity (TWIP) . Prevailing theory suggests cold work nucleates twinning, twinning growth occurs as deformation increases, mean free path is reduced, and dislocation glide is altered. Lower MR artifact indicates that better diagnostic imaging is possible with Mo-47.5Re implants. Fatigue results indicate that downsized implant dimensions may be designed as a result of the unique mechanical properties which can compensate for the high density and modulus.
References:
1 Standard Specification for Wrought Molybdenum-47.5 Rhenium Alloy for Surgical Implants (UNS R03700).
2 S. Agnew and T. Leonhardt, (undated) Texture, Anisotropy and the Role of Twinning in Determining the Mechanical Behavior of a Molybdenum-Rhenium Alloy, Rhenium Alloys Abstract No.19, p.7.
MiRus™ Spine Alignment Monitoring System Utilizing Miniaturized Wireless MEMS Sensors Provides real-time, Precise and Safe Measurement of Intra-opertive Spine Alignment
Larson J, et al.
North American Spine Society (NASS) Annual Meeting,
October 26, 2017
Introduction: Sagittal alignment of the spine is closely related to quality of life scores and malalignment is a cause of pain and disability. Current methods of intra-operative measurements are inadequate for real-time measurement of segmental and overall spinal alignment. The objective of this study was to validate a wireless, non-optical system for dynamic assessment of spine alignment during surgery.
Methods: The intra-operative spine alignment monitoring system (SAM) utilizes disposable, miniaturized wireless MEMS sensors, advanced signal processing, and error compensation algorithms to provide dynamic measurement of sagittal plane intervertebral angles and overall spinal alignment. The sensing module is battery-powered and communicates information wirelessly to the display unit. Registration is performed with a single fluoroscopic image. The goal is to provide continuous tracking of spine alignment without reliance on repeated imaging.
System accuracy was validated via benchtop and simulated use-testing. Benchtop testing simulated motions that could be encountered during surgery using precision stages that allowed for accurate reference values for angular measurements. Simulated clinical use-testing using radiopaque Sawbones® phantoms compared manual Cobb angle measurements of Lordosis and Kyphosis on lateral fluoroscopic images to the values measured by the SAM.
Results: In benchtop testing, mean difference between the reference values and the spine alignment monitoring system measurements was 0.0 degrees with a standard deviation of 0.9 degrees (n= 90). In simulated use testing, mean difference between the cobb angle measurements and the spine alignment monitoring system measurements was 1.5 degrees with a standard deviation of 1.9 degrees (n = 30).
Conclusion: Benchtop and simulated use testing of the SAM demonstrated high accuracy and repeatability. This approach could provide surgeons with practical, real-time measurements of segmental and overall alignment, thus allowing attainment of sagittal balance.
Mechanically and Biologically Superior Molybdenum Rhenium (MoRe®) Alloy Provides an Advanced Option for Spinal Implants
Poelstra K, et al.
North American Spine Society (NASS) Annual Meeting
October 25, 2017
Background Context: Spine surgery has improved dramatically over the past decades primarily due to the development of better techniques but little progress has been made in the development of new materials for spine implants. Grade 23 Titanium (Ti-6Al-4V ELI, “Ti-ELI”), the most commonly used alloy for spinal implants, has a good biological profile but has limited strength and is notch-sensitive so repetitive stress leads to fatigue failure of implants and limits design options. Cobalt Chromium (CoCr) provides enhanced strength without an increase in the size of implants, however, it has an inferior biological response and remains sensitive to fatigue and infection. Better materials with optimized biomechanical and biological properties could result in the development of superior spinal implants and surgical techniques.
Purpose: To evaluate the mechanical and biological properties of Molybdenum-Rhenium (MoRe®) for spinal implants.
Methods:
Multiple tests were performed to evaluate the mechanical and biological properties of MoRe®.
- Standard test methods (ASTM 1717) for Spinal Implant Constructs in a Vertebrectomy Model were used to evaluate the Molybdenum Rhenium (MoRe®) alloy compared to Ti-6Al-4V (ASTM F136-13 annealed bar, Ti-ELI) and CoCr (ASTM F1537-11 warm worked).
- A bone implantation study was conducted based on ISO 10993: Biological Evaluation of Medical Devices, Part 6: Tests for Local Effects after Implantation to evaluate the local tissue and bone response of MoRe® alloy test coupon compared to Ti-ELI controls, when implanted in the mid-shaft femoral bone of rabbits for 4, 13 and 26 weeks.
- Biofilm formation study was conducted to quantify attachment and biofilm development of S. aureus bacteria upon MoRe® alloy in comparison to Ti-ELI, CoCr, and Stainless Steel in a well-controlled, reproducible, ex vivo laboratory experiment.
Results: MoRe is composed purely (99.99%) of molybdenum and rhenium and does not contain Nickel. Molybdenum is found in food and is a cofactor to the enzymes xanthine oxidase and sulfite oxidase, which are essential to bone and connective tissue metabolism. Rhenium is an inert metal with no biological affect. Mechanical testing showed:
Yield Strength: MoRe® 280ksi, Ti-ELI 115ksi, CoCr 110ksi.
Ultimate Tensile Strength: MoRe® 300ksi, Ti-ELI 125 ksi, CoCr 170 ksi.
Elongation and Reduction in Area: MoRe® 13%, 50%, respectively; T-ELI 10%, 25%; CoCr 12%, 12%.
Recoil: MoRe® <2%, Ti-ELI 6%, CoCr 9%.
Hardness Range: MoRe® 280-800HV, Ti-ELI 350-400HV, CoCr 255-331HV.
Max Run-Out Load Bent Rod: MoRe® 4.0mm rod 350N, Ti-ELI 5.5mm rod 150N, CoCr 5.5mm rod 200N.
Decrease in Max Run-Out Load Bent, Unbent, Re-bent Rod: MoRe® -9%, Ti-ELI -17%, CoCr -13%.
The bone implantation study demonstrated continued bone maturation over time for both MoRe ®and Ti-ELI implant sites with similar osteoconduction and bone remodeling histology of the cortical and medullary bone at all time points. Biofilm growth on MoRe® was less than CoCr (p = 0.009) and Stainless Steel and similar to Ti-ELI.
Conclusions: The MoRe® alloy, with its advantageous mechanical and biological properties, offers great promise for the design of a new generation of smaller, more fatigue resistant and bio-friendly spine implants, resulting in less soft tissue disruption, quicker recovery and better outcomes for patients.
Real-time 3D Navigation of Rod Insertion and Passage in MIS Spine Surgery
Dorchak J, et al.
North American Spine Society (NASS) Annual Meeting. October 25, 2017
Background Context: Rod sizing and insertion can be difficult to achieve and be a source of surgeon frustration even in one and two level MIS cases. The use of navigation in MIS spine surgeries has the potential of improving implant placement speed and accuracy as well as reducing surgeon and staff exposure to ionizing radiation. Current navigation tools are limited and do not allow navigation of rod placement.
Purpose: MiRus™ has developed an innovative navigation system that provides real-time guidance during rod insertion. Using miniaturized fiducials that attach to standard MIS instrumentation and a small camera placed near the surgical field, the system tracks the position of the rod relative to the tulip heads. The information is presented as a live 3D view. Distance between the tulip heads is measured and monitored allowing the surgeon to accurately size rod length. The system addresses the key shortcomings of traditional navigation systems such as large fiducials and cumbersome registration.
Study Design/Setting: A key technological feature of the system is the ability to track the rods and towers using fiducials. A bench set-up using a representative tool was created and testing was performed by comparing measurements by the system to those by an independent reference system.
Methods: The orientation and tip position of a representative rod insertion tool affixed with a fiducial was measured by the MiRus system and compared to measurements of the same by a reference system. Translational and rotational movements were measured.
Results: Dynamic testing of the orientation of the representative rod insertion tool found a mean error of 0.35mm with a standard deviation of 1.50mm (n=24). The stationary tip test used to measure tip position, resulted in standard deviations of 1.7mm in the x-axis, 1.8mm in the y-axis and 0.63mm in the z axis (n=200). The performance of the MiRus Rod Navigation System was within the clinical requirement of +/- 2mm for rod navigation.
Conclusion: Feasibility of real-time rod navigation was demonstrated using the MiRus Navigation System. Using miniaturized fiducials and advanced computer vision techniques, the MiRus system is able to facilitate this critical step in MIS spine surgery.
A Wireless, Non-optical System for Measuring Intra-operative Spine Alignment
Larson J, Poelstra K.
Society for Minimally Invasive Spine Surgery (SMISS) Annual Meeting September 16, 2017
Objective: To assess the feasibility and performance of a new non-optical, wireless navigation system for dynamically assessing spine alignment during surgery.
Introduction: Sagittal alignment of the spine is closely related to quality of life scores and malalignment is a cause of pain and disability. Stabilization of the spine and restoration of normal posture and spine alignment using osteotomies and/or fusion is a major goal of spine surgery.
Current methods of intra-operative measurements are inadequate, cumbersome and not dynamic. Limitations include increased intraoperative exposure to radiation (fluoroscopy, CT) and inadequate software tools. Technological limitations of current spine navigation systems – such as large size of tracker arrays, high cost of capital equipment, and line of sight issues – make them unsuitable for real-time measurement of segmental and overall spinal alignment.
Methods: An intra-operative spine surgery system was developed which utilizes disposable, miniaturized wireless MEMS (micro-electromechanical) sensors, advanced signal processing and error compensation algorithms to fuse gyroscopic, magnetic and acceleration data and provide accurate real-time measurement of 3D vertebral orientation and spinal alignment. Each wireless sensing module (WSM) is battery-powered and communicates information wirelessly to the display unit. The goal was to provide continuous tracking of spinal alignment without reliance on repeated imaging. Registration is performed with a single fluoroscopic image.
Performance testing of the WSM was performed using a high precision motion controller with a resolution of 0.0002 degrees. The WSM is tested for orientation measurement accuracy for rotations about its X, Y, and Z axes. Both the accuracy and repeatability of the orientation measurements were evaluated.
Results: During dynamic testing after a single initial registration, the WSM had mean errors of -0.06 degrees, 0.19 degrees, and 0.15 degrees and standard deviations of 0.13 degrees, 0.32 degrees, and 0.17 degrees about its X, Y, and Z axes respectively. The performance of the WSM was well within the clinical requirement of +/- 3 degrees for spinal alignment measurement.
Conclusion: A low cost, non-optical, disposable wireless system for dynamic measurement of intra-operative spine alignment had high precision and accuracy in bench top testing. This approach could provide surgeons with practical, real-time measurements of segmental and overall alignment, thus allowing attainment of sagittal balance.
Molybdenum-Rheium (MoRe®) Alloy as a Superior Alloy for MIS Spinal Implants
Poelstra K, Larson J.
Society for Minimally Invasive Spine Surgery (SMISS) Annual Meeting. September 16, 2017
Introduction: Spine surgery has improved dramatically over the past decades primarily due to the development of better techniques but little progress has been made in the development of new materials for spine implants. Better materials with optimized biomechanical properties could result in the development of superior MIS implants and surgical techniques.
Aims/Objectives: To evaluate the feasibility of a new, stronger, material for MIS Spinal Implants.
Methods:
- Standard test methods (ASTM 1717) for Spinal Implant Constructs in a Vertebrectomy Model were used to evaluate the Molybdenum Rhenium (MoRe®) alloy compared to Ti-6Al-4V (ASTM F136-13 annealed bar, Ti-ELI) and CoCr (ASTM F1537-11 warm worked).
- A bone implantation study was conducted based on ISO 10993: Biological Evaluation of Medical Devices, Part 6: Tests for Local Effects after Implantation to evaluate the local tissue and bone response of MoRe® alloy test coupon compared to Ti-ELI controls, when implanted in the mid-shaft femoral bone of rabbits for 4, 13 and 26 weeks.
- Biofilm formation study was conducted to quantify attachment and biofilm development of S. aureus bacteria upon MoRe® alloy in comparison to Ti-ELI, CoCr, and Stainless Steel in a well-controlled, reproducible, ex vivo laboratory experiment.
Results: MoRe® is composed purely (99.99%) of molybdenum and rhenium and does not contain Nickel. Molybdenum is found in food and a fundamental component of bone. Rhenium is an inert metal with no biological affect. Mechanical testing showed:
Yield Strength: MoRe® 280ksi, Ti-ELI 115ksi, CoCr 110ksi.
Ultimate Tensile Strength: MoRe® 300ksi, Ti-ELI 125 ksi, CoCr 170 ksi.
Elongation and Reduction in Area: MoRe® 13%, 50%, respectively; T-ELI 10%, 25%; CoCr 12%, 12%.
Recoil: MoRe® <2%, Ti-ELI 6%, CoCr 9%.
Hardness Range: MoRe® 280-800HV, Ti-ELI 350-400HV, CoCr 255-331HV.
Max Run-Out Load Bent Rod: MoRe® 4.0mm rod 350N, Ti-ELI 5.5mm rod 150N, CoCr 5.5mm rod 200N.
Decrease in Max Run-Out Load Bent, Unbent, Re-bent Rod: MoRe® -9%, Ti-ELI -17%, CoCr -13%.
Bone implantation study demonstrated continued bone maturation over time for both MoRe® and Ti-ELI implant sites with similar osteoconduction and bone remodeling histology of the cortical and medullary bone at all time points. Biofilm growth on MoRe® was less than CoCr (p = 0.009) and Stainless Steel and similar to Ti-ELI.
Conclusions: MoRe® has superior mechanical and biological properties compared to current implant materials and offers great promise for the design of a new generation of smaller, MoRe® fatigue resistant and bio-friendly implants, ideal for MIS spine applications.
